Ontario Animal Health Network (OAHN) Bovine Expert Network Quarterly Veterinary Report
Surveillance Updates
Ontario: Bovine Theileriosis
In October 2025, a case of bovine theileriosis due to Theiliera orientalis Ikeda was detected in a dairy cow imported from the United States. Veterinarians are urged to familiarize themselves with the disease so they can make recommendations for preventative measures on high-risk farms, include as a differential diagnosis, and recommend testing where appropriate.
Risk to Ontario farms is higher for open herds, and especially for farms importing cattle. The prevalence of Theileria orientalis Ikeda in the United States is largely correlated with the presence of the Asian Longrhoned Tick (ALHT). The most updated American map can be found at Longhorned-Tick-Situation Report
An overview of the Ontario finding can be found at Veterinary advisory: bovine theileriosis (November 12, 2025) | Animal health updates and veterinary advisories | ontario.ca
Check out 2 new OAHN Resources:
1) Factsheet: Asian Longhorned Tick – An Encroaching Threat
2) Factsheet: Bovine Theileriosis
Other Resources: Animal Health Canada ALHT Infographic
Quebec: Bovine Anaplasmosis
A case of bovine anaplasmosis was confirmed in a 10-year-old beef cow from a cow-calf farm in Quebec in the fall of 2025. Signs of anemia and jaundice were detected in six cows on the farm; five out of 21 adult cattle died, and the positive cow was euthanized. An epidemiological investigation has been undertaken and control measures have been implemented on the farm.
United States: Highly Pathogenic Avian Influenza (HPAI) in Dairy Cattle in Wisconsin
In December, HPAI was confirmed in a Wisconsin dairy herd. This is the first confirmed case in the State and was confirmed by USDA to be a new wildlife spillover of the D1.1 strain, separate from earlier outbreaks. This marks the fourth spillover from birds to cattle, previously two D1.1 spillover events were detected in Nevada and Arizona dairy herds and the original spillover event occurred in the Texas Panhandle in late 2023 involving the B3.13 strain.
Q3 Bovine Data from the Animal Health Laboratory
Pathology Cases of Interest
Vascular Hamartoma in an Aborted Fetus
History: 2nd abortion in 4 months, fetus submitted for diagnostic work-up.
Pathology: Grossly, the late gestation Holstien fetus had tri-cavitary effusion, the liver was nodular and fibrotic, and the heart was globoid with flaccid ventricular walls that were expanded by multiple blood-filled spaces, which were microscopically confirmed to be vascular proliferations. In the liver, there was marked portal bridging fibrosis with biliary hyperplasia, in addition to fibrosis surrounding dilated central veins and dissecting between and individualizing adjacent hepatocytes.
Summary: Vascular hamartomas are infrequently reported in the heart (or other organs) of calves at slaughter, and these benign malformations are not typically associated with clinical disease. In this case, the extensive involvement of the ventricular and intraventricular myocardium severely compromised fetal heart function resulting in congestive heart failure.
Clostridium septicum associated with Recent Dehorning
History: 1-month-old dairy calf, side of face swollen, dehorned earlier last week. Euthanized.
Pathology: Grossly, extending from near the ventral margin of the dehorning sites on both sides, the subcutaneous tissues were mildly thickened, firm, and contained turbid blood-tinged edema that dissected down around the regional salivary glands, muscle bundles, trachea, and esophagus. Microscopic examination confirmed the presence of cellulitis with proteinaceous edema and suppurative inflammation, which also dissected into adjacent skeletal muscle and adipose tissue.
Ancillary testing: Clostridium septicum was isolated from the affected tissue, along with Helcococcus ovis
Summary: This calf had regionally extensive suppurative cellulitis associated with recent dehorning sites. Clostridium septicum was isolated from the affected tissue, which along with the gross and microscopic findings, fits with a diagnosis of Clostridial malignant edema. Helcococcus ovis was also isolated, and this is probably indicative of an opportunistic coinfection.
Stats for Q3
- A total of 1668 bovine cases were submitted to the AHL during Q3, spanning from August 1 2025 to October 31 2025.
- Of these, 165 submissions had a pathology component, consisting of 47 postmortem cases and 118 send-in cases.
- 13 reproductive loss investigations
- 24 young calves (under 2 months of age)
- 50 older calves (2 months to 2 years of age)
- 34 adult cattle
- 44 meat inspection cases
- Submissions from practitioners included 72 dairy, 41 beef, 1 bison, and 7 where the commodity was not specified. Insufficient clinical history was associated with 4 submissions.
- A total of 99 submissions had a definitive or presumptive diagnosis, and 22 did not have a specific diagnosis (5 abortion work-ups, 1 young calf, 10 older calves, 6 adults).
- Most of the cases with no specific diagnosis were send-in. For several submissions, a semi-comprehensive or complete set of tissues had not been sampled for histopathology (including brain), and detailed necropsy findings were not conveyed.
SALMONELLA
In total, 185 bovine submissions had bacterial culture performed (non-milk), generating 453 cultures. Salmonella spp. were isolated from 10 submissions, representing an estimated 9 premises.
- Salmonella Dublin was isolated from 6 submissions, and 5 of these have no noted record of previous isolation of this pathogen at the premises.
- 1-year-old dairy animal: Suspect Salmonella (feces submitted)
- 4-month-old dairy calf: Anorexia, weak/lethargic, recent arrival from USA, postmortem identified mild enterocolitis and rumenitis (intestine)
- No history provided (lung)
- 2-month-old dairy calf: Increase in calves with septic joint disease responding poorly to treatment (joint swab)
- 2-month-old dairy calves: septicemia
- 3-month-old beef calves: Calves scouring between 7 to 10 weeks of age, septicemia (lung)
- Salmonella Muenster (1 submission: send-in)
- Dry cow found dead, isolated from lung; compatible with septicemia
- Salmonella Newport (3 submissions: all fecal samples for bovine neonatal enteric panel)
- 2-month-old calf died, NAF on-farm necropsy, history of scours on farm. Isolated from enrichment, no cryptosporidium or rotavirus or coronavirus detected.
- 1-month-old calf died, history of scours on farm. No cryptosporidium or rotavirus or coronavirus detected.
- 10-day-old scouring calf. Isolated from enrichment, along with 3+ E. coli. No cryptosporidium. Rotavius A and coronavirus detected with Ct values of approximately 30.
There were 11 bovine submissions that had Salmonella Dublin PCR performed, generating 2 positive submissions (2-month-old beef calves and a 2-month-old dairy calf, all with presumed septicemia).
BOVINE VIRAL DIARRHEA VIRUS (BVDV)
In total, 47 submissions generated 172 samples for BVDV PCR testing. This yielded 3 positive samples (BVDV type I) from a single submission (blood samples with no history provided). BVDV immunohistochemistry was not performed for any pathology submissions.
This summary has been compiled by Dr. Rebecca Egan, Animal Health Laboratory (AHL) from diagnostic submissions to the AHL Guelph and Kemptville locations.
OAHN Project Summary: Investigation of Mycoplasma wenyonii and Candidatus Mycoplasma haemobos in Ontario dairy cattle
Background: Mycoplasma wenyonii and Candidatus Mycoplasma haemobos are cattle-specific members of the hemoplasmas and recognized as hemotrophic, epicellular bacterial parasites that attach to the surface of red blood cells. M.wenyonii has been recognized in sick animals exhibiting a range of clinical signs, most commonly hind limb and mammary edema, pyrexia, enlarged prefemoral lymph nodes, and general icterus associated with hemolytic anemia. Prolonged infection has been linked to lower milk yield, reduced calf birth weights, and bull infertility. Importantly, M.wenyonii has also been detected in apparently healthy animals with subclinical infection. M. wenyonii can be transmitted by iatrogenic blood transfer (e.g., re-use of contaminated needle) or by vectors such as lice, flies, ticks and mosquitoes. Vertical transmission in utero has also been reported. Candidatus Mycoplasma haemobos, another hemotrophic Mycoplasma spp. has been shown to cause anemia and depression with similar risks for transmission as M. wenyonii.
Prevalence: M. wenyonii has been recognized worldwide. A prevalence study in Michigan and Wisconsin in 2018 found an estimated within-herd apparent prevalence of 71.7% ± 1.0% for M. wenyonii and 77.3% ±1.0% for C.M. haemobos, indicating infection is likely endemic. To date, there have been no published prevalence studies in Canada, although these organisms have been identified in clinical outbreaks on dairy farms in Ontario.
Objective: To conduct a pilot study of blood samples collected from Ontario dairy cattle and determine whether M. wenyonii and C. M. haemobos are present in Ontario dairy herds, and to explore potential herd-level risk factors.
Methods: This study was completed at 10 commercial dairy farms in Southwestern Ontario from December 2023 to May 2024. A blood sample was collected into an EDTA tube from 30 early-lactation cows per farm. Samples were submitted to the Animal Health Laboratory (Guelph) for gel-based PCR testing for M. wenyonii and the C. M. haemobos. A questionnaire was completed with each herd regarding herd characteristics and management practices such as herd size, animal purchases, outdoor access, insect control practices, needle use, and calf housing.
Results and Discussion:
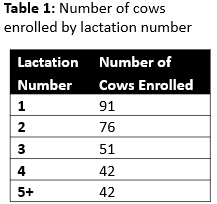
Producers already participating in an unrelated project were approached for recruitment. No herds had clinical signs suspicious for hemotrophic mycoplasmosis. The number of lactating cows on the enrolled farms ranged from 67 to 628. A total of 297 dairy cattle were tested, with all samples collected from animals between 21 and 28 days in milk. Parity was recorded and is described in Table 1.
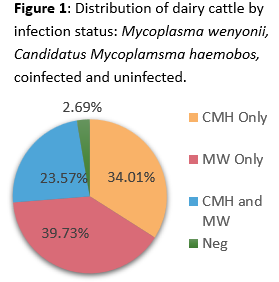
 The PCR-positive proportions were 63.3% and 54.6% for Mycoplasma wenyonii and Candidatus Mycoplasma Haemobos, respectively. Seventy cows (23.6%) tested positive for both organisms. With the overlap between positive animals, only 2.7% of cattle tested were negative for both hemoplasmas (Figure 1). Blood smears were assessed by a clinical pathologist on a subsample of positive cattle (n=28). No hemotropic mycoplasma organisms were identified in any of the samples, reinforcing the low sensitivity of blood smear evaluation for these infections.
The PCR-positive proportions were 63.3% and 54.6% for Mycoplasma wenyonii and Candidatus Mycoplasma Haemobos, respectively. Seventy cows (23.6%) tested positive for both organisms. With the overlap between positive animals, only 2.7% of cattle tested were negative for both hemoplasmas (Figure 1). Blood smears were assessed by a clinical pathologist on a subsample of positive cattle (n=28). No hemotropic mycoplasma organisms were identified in any of the samples, reinforcing the low sensitivity of blood smear evaluation for these infections.
 While this was not a large enough sample size for a formal prevalence estimate, given the high proportion of PCR-positive animals and the endemic status of nearby U.S. states, it is likely these hemotrophic Mycoplasma species are widespread in dairy cattle in southwestern Ontario. To date, Mycoplasma wenyonii has only rarely been diagnosed and associated with clinical disease on farms in Ontario, but limited awareness of symptoms and limited testing may contribute to under-recognition. The association of subclinical infection with health and welfare is poorly understood. Hemoplasma infection alone does not usually result in clinical disease, and most animals remain apparently healthy. However, researchers have hypothesized that subclinical infection with hemotrophic mycoplasmas might enhance susceptibility to or severity of other diseases.
While this was not a large enough sample size for a formal prevalence estimate, given the high proportion of PCR-positive animals and the endemic status of nearby U.S. states, it is likely these hemotrophic Mycoplasma species are widespread in dairy cattle in southwestern Ontario. To date, Mycoplasma wenyonii has only rarely been diagnosed and associated with clinical disease on farms in Ontario, but limited awareness of symptoms and limited testing may contribute to under-recognition. The association of subclinical infection with health and welfare is poorly understood. Hemoplasma infection alone does not usually result in clinical disease, and most animals remain apparently healthy. However, researchers have hypothesized that subclinical infection with hemotrophic mycoplasmas might enhance susceptibility to or severity of other diseases.
Clinical Implications for Ontario Veterinarians
Ontario veterinarians should consider testing for Mycoplasma wenyonii and C.M haemobos to support diagnosis in compatible clinical cases (e.g., anemia, edema, fever, or unexplained production losses) and guide case management decisions .
As always, testing results should be interpreted alongside the clinical presentation, a complete blood count (CBC; especially if anemia is present), and the herd history and seasonality (M. wenyonii is often worse in summer/fall). In Canada, Mycoplasma wenyonii and C.M. haemobos are not reportable. Testing is available through the Animal Health Laboratory (Guelph) in Ontario.
Tips for Testing for Mycoplasma wenyonii and Candidatus Mycoplasma haemobos
1. Use PCR on whole blood
This is the diagnostic test of choice. M. wenyonii and C. M Haemobos cannot be reliably cultured using routine bacterial methods. Blood smears are unreliable. While Giemsa‑ or Wright‑stained blood smears may occasionally show organisms, sensitivity is very low. Blood smears should not be used to rule in or rule out infection, PCR is required.
2. Sample type and handling
Submit EDTA whole blood. The best sample is at least 1-2 ml of whole blood in a purple‑top tube (EDTA). Avoid clotted samples, as M. wenyonii attaches to red blood cells. Samples should be refrigerated and shipped within 24-48 hours.
3. Animals to sample
Sample clinically affected animals first, as organism load is highest during acute clinical signs. Testing yield is highest when sampling:
- Febrile cows
- Cows with sudden milk drop
- Animals with udder, brisket, or limb edema
- Anemic animals (pale mucous membranes)
If investigating a herd problem, sample 5–10 clinically affected animals and 2–3 apparently healthy animals for comparison. Chronic or recovered animals may test PCR‑negative despite prior infection.
4. Consider co-morbidities and differentials
M. wenyonii often occurs alongside other conditions and may be one of several differential diagnoses. Depending on clinical signs, consider also testing for:
- Anaplasma marginale
- Theileria orientalis Ikeda
- Bovine viral diarrhea virus (BVDV)
- Bovine leukemia virus (BLV, if anemia or immunosuppression suspected)
- Mastitis pathogens (if udder edema or milk changes dominate)
M. Wenyonii References:
1. Strugnell BW, McAuliffe L. Mycoplasma wenyonii infection in cattle. In Pract 2012:34;146-154.
2. Tagawa M, Yamakawa K, Aoki T, et al. Effect of chronic hemoplasma infection on cattle productivity. J Vet Med Sci 2013;75:1271-1275.
3. Schambow RA, Poulsen K, Bolin S, Krahn D, Norby B, Sockett D, Ruegg PL. Apparent prevalence of Mycoplasma wenyonii, Candidatus Mycoplasma haemobos, and bovine leukemia virus in Wisconsin and Michigan dairy cattle herds. JDS Commun. 2021 Jan 22;2(2):61-66.


