Ontario Animal Health Network (OAHN) Poultry Expert Network Quarterly Veterinarian Report
Highly pathogenic avian influenza (HPAI) H5N1 – What mammals are affected?
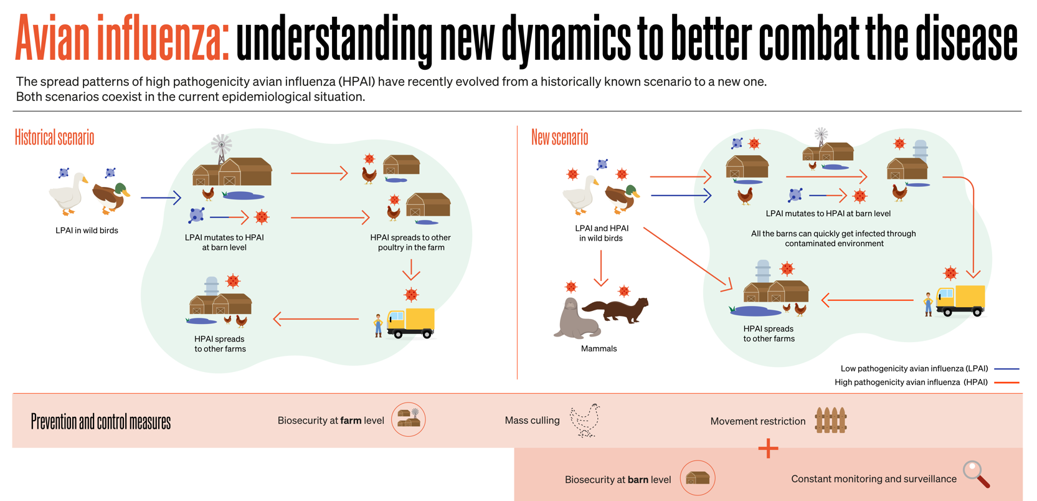
Dr. Emily Martin, Animal Health Laboratory, University of Guelph As cases of HPAI H5N1 continue to be detected it is apparent that the evolving situation is much different than previous outbreaks. For years it has been generally accepted that influenza viruses evolve by circulating between birds, swine and humans resulting in recombination of genetic material. When low pathogenic avian influenza (LPAI) H5 and H7 viruses gained access to poultry flocks they had the potential to convert to HPAI causing increased mortality requiring stamping out measures. By implementing movement control and monitoring, an outbreak could be limited. However, since 2020 the currently circulating H5N1 virus has been operating in a different environment, primarily due to wide circulation within wild bird populations and affecting a wider variety of bird species. This has allowed the virus increased opportunity to spill over into domestic and other wildlife species including a vastly expanded variety of mammalian species than previously identified. The World Organization for Animal Health (WOAH, founded as OIE) illustrates the differences in how influenza circulates in the follow historical versus new scenarios:
Based on a recent literature search, the following 2 tables compare the list of mammalian species infected with influenza viruses in previous outbreaks versus the current outbreak. The lists show a wider diversity of species affected in the current outbreak that are also from a larger geographic area. (Note: Tables are not an exhaustive list of affected species.) Some species are more commonly identified as being affected or have experienced mass mortality events (e.g. >1,000 cases: American sea lions, American mink and arctic fox). Also, carnivorous species are over represented, likely due to scavenging activities on affected carcasses. Table 1. Mammals previously reported to be infected with H5N1 (prior to 2019) From 10 countries (5 Asia, 3 Europe, 2 Africa)
| Domestic | Carnivore | Omnivore | Herbivore |
| Cats | Tiger | Raccoon dog | Plateau pikas |
| Dogs | Leopards | Stone marten | |
| Pigs | Lion | Owston’s civet | |
| Donkey | Mink |
Table 2. Mammals additionally reported to be infected with H5N1 (current outbreak) From 26 countries (17 Europe, 5 South America, 2 North America, 2 Asia)
| Domestic | Carnivore | Omnivore | Herbivore |
| Cat | South American bush dog | Red fox | Beaver |
| Dog | Coyote | Arctic fox | Abert’s squirrel |
| Ferret | Eurasian otter | Grey fox | Eastern Grey squirrel |
| Dairy Milking Cows | Marine otter | Badger | Prairie vole |
| Swine | North American river otter | Fisher cat | Desert cottontail |
| Alpaca | Bobcat | Stone marten | |
| Lynx (Canada, Eurasian) | Pine marten | ||
| Tiger | American marten | ||
| Amur tiger | Raccoon | ||
| Hybrid tiger | South American coati | ||
| Bengal Tiger | Skunk (Striped) | ||
| Mountain lion | Asiatic black bear | ||
| African Lion | American black bear | ||
| Caracal | Grizzly bear | ||
| Leopard | Kodiak bear | ||
| Amur leopard | Virginia opossum | ||
| Geoffroy’s cat | Muskrat | ||
| Savannah cat | House mouse | ||
| Serval | Deer mouse | ||
| Mink (American) | Black rat | ||
| Ermine | Norway rat | ||
| Long-tailed weasel | Raccoon dog | ||
| Grey seal |
| Harbor seal | |||
| Caspian seal | |||
| Southern elephant seal | |||
| South American fur seal | |||
| American sea lions | |||
| Burmeister’s porpoise | |||
| Porpoise | |||
| Chilean dolphin | |||
| White-sided dolphin | |||
| Common dolphin | |||
| Bottlenose dolphin | |||
| Polar bear |
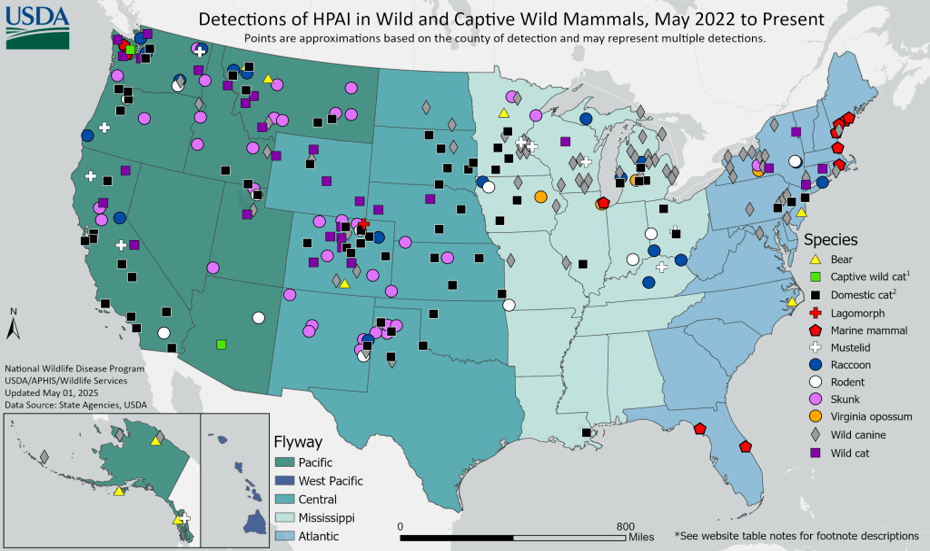
Infected mammals can be subclinical (no observable signs) or present with respiratory or neurological signs (e.g. ataxia, tremors, seizures). An example of domestic species being infected with H5N1 was identified at AHL in March of 2023 when a Golden retriever became ill and died after scavenging on a Canada goose carcass. Mammals are usually considered dead end hosts. However, it is still questionable if the virus can pass from mammals back to birds depending on if there are mutations within the circulating virus. The following map shows HPAI detections in mammals throughout wild bird flyways in the USA. There are also cases of mammals being fed raw poultry meat infected with the virus becoming ill.
As H5N1 continues to circulate in a wide variety of species, the opportunity for the virus to mutate and spill over into more species continues to evolve. The World Organization for Animal Health has recommendations for how to protect poultry flocks (https://www.woah.org/app/uploads/2023/09/avian-influenza-farmers-protect-your-poultry-from-avian-influenza.pdf), however, knowing that the virus is circulating in birds and mammals should be kept in mind when evaluating potential sources of risk.
References:
1) PI, Gamarra-Toledo V, Euguí J, et al. Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide. Emerging Infectious Diseases. 2024;30(3):444-452. https://doi.org/10.3201/eid3003.231098
2) OFFLU call to discuss Avian Influenza events in mammals 2 March 2023 https://www.offlu.org/wp-content/uploads/2023/03/OFFLU-call-AI-mammals-Mar2023.pdf
3) Infection with highly pathogenic avian influenza (HPAI) H5N1 in an Ontario dog. https://www.uoguelph.ca/ahl/infection-highly-pathogenic-avian-influenza-hpai-h5n1-ontario-dog
Poultry Veterinarian Survey Highlights – Q1 2025 (Dec 2024 – Feb 2025)
Broilers
Practitioners
Stable conditions: salmonellosis, chicken anemia virus (CAV), necrotic enteritis, lameness developmental (tibial dyschondroplasia) and nutritional, coccidiosis, spiking mortality, infectious bronchitis (IBV), infectious bursal disease (IBVD), condemnation issue (cellulitis due to bacterial lameness, chronic respiratory disease) and avian influenza.
Stable to decreased conditions: runting stunting syndrome (RSS) and avian metapneumovirus (aMPV) infections.
Stable to increased conditions: infectious laryngotracheitis, early systemic bacterial infection (E. coli and E. cecorum), other causes of mortality (dehydration, fungal airsacculitis and fungal pneumonia), late systemic bacterial infection (E. coli and E. cecorum), lameness of bacterial origin (E. coli and E. cecorum), ascites and inclusion body hepatitis.
Increased to equal stable or decreased conditions: lameness of viral origin. E. coli resistance to TMS was reported at 10-24% this quarter. Below graph depicts the frequency of the conditions seen in the flocks for the past 3 quarters. The most frequent 3 conditions seen this quarter were: early systemic bacterial infection, late systemic bacterial infection and lameness of bacterial origin.
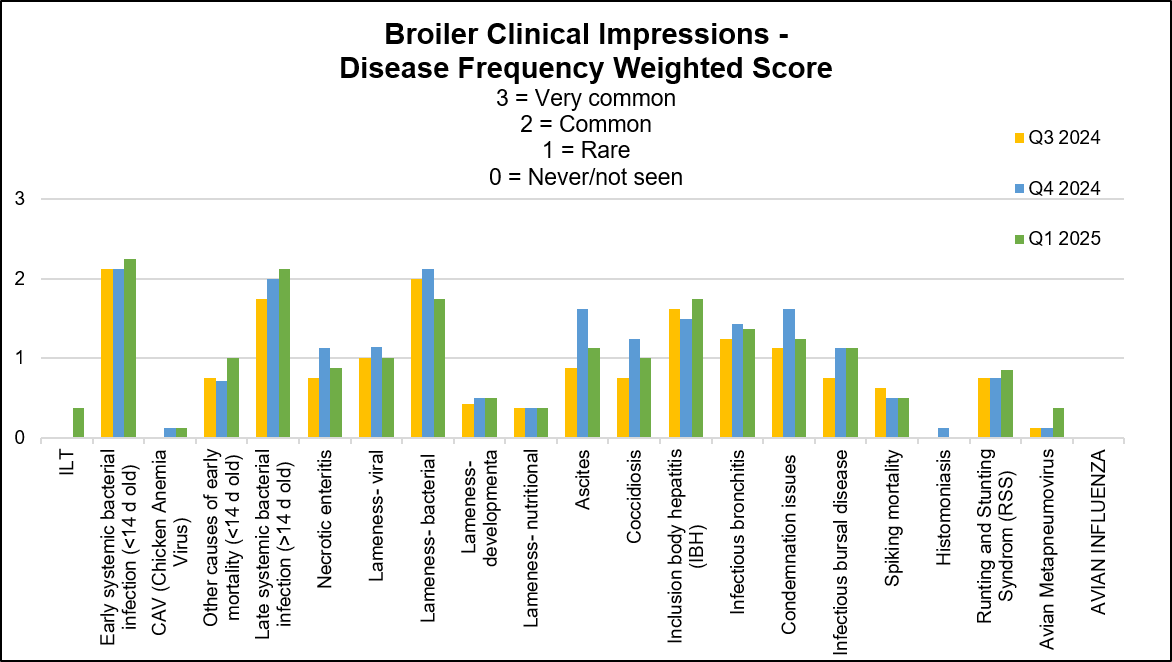
AHL
Similar number of cases (from previous quarter):
- Early systemic bacterial infection (< 14 days old) – All cases had E. coli isolated.
- Lameness viral (Reovirus confirmed: SK_R12, BC-17-0408-2017, Georgia 13-097066, ON_variant_A_12-073195, ON_variant_E_12-090746, ON_variant_F_12-087411, ON_variant_G_16-102019 and ON_variant_H_18-008168).
- Necrotic enteritis.
- Inclusion body hepatitis (FAdVE, FAdVE/FAdVD, FAdVE/FAdVAC, FAdVE/FAdVD/FAdVAC, FAdV08b_AHL_16-049095_ON, FAdV08b_AHL_18-057921_ON).
- Infectious bursal disease (PCR positive field strains: USA PA105-2014, British Columbia 15-062782, USA Del-E and USA 66-Indiana-2014. Vaccine strains: USA W2512-Blen and USA D78).
Increased number of cases (from previous quarter):
- Other causes of mortality (> 14 days old) – Mycotic pneumonia and airsacculitis (E. coli and E. cecorum).
- Lameness viral (Reovirus suspicious, primarily diagnosed on histology).
- Infectious bronchitis (IBV – pathology cases and PCR positive)( Field strains: IBV_DMV_ON_21-017385 and IBV_DMV_ON_15-077145. Vaccine strains: IBV_Conn, IBV_Mass-MA5 vaccine and USA Mass-AHL 21-008165 vaccine.)
- ILT Niagara-like (CAGG)(1 case)
Decreased number of cases (from previous quarter):
- Late systemic bacterial infection (> 14 days old) – E. coli or E. cecorum isolated either in pure culture or in combination.
- Lameness nutritional (rickets).
- Lameness developmental (tibial dyschondroplasia).
- Lameness bacterial (Primarily diagnosed on histology) – E. coli or E. cecorum isolated either in pure culture or in combination. Also E. coli isolated with S. Infantis. Cases of osteomyelitis have E. coli isolated alone or in combination with E. cecorum.
- Coccidiosis (small intestine).
- RSS – Confirmed.
No cases diagnosed this quarter:
- Avian metapneumovirus (Type B).
- RSS – Suspect.
- Spiking mortality.
- Dead on arrival (DOA).
Other diagnostic findings:
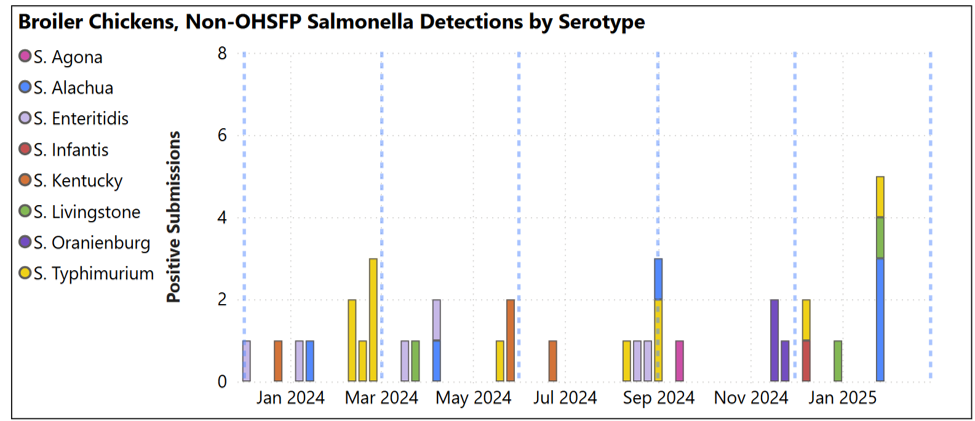
Tibial rotation, liver hemorrhage, heart failure/right ventricular dilation, heterophilic typhlitis / enteritis with adherent bacteria, nasal sinusitis with intralesional bacteria, pneumonia, myocarditis, bursitis, dorsal cranial myopathy and lymphoid neoplasia. Salmonella: Practitioners: The most common serotypes isolated were serotypes D with S. Kentucky listed with the clinical impression. Animal Health Laboratory Salmonella Isolations: Broilers (blue dashed lines indicate quarter periods: Dec-Feb, Mar-May, Jun-Aug, Sept-Nov).
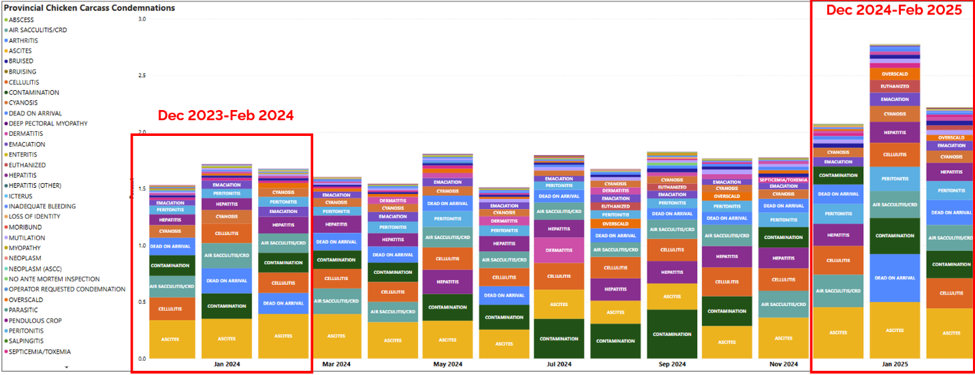
Condemnation conditions in provincial slaughter plants reported for period of December 2023-February 2025.
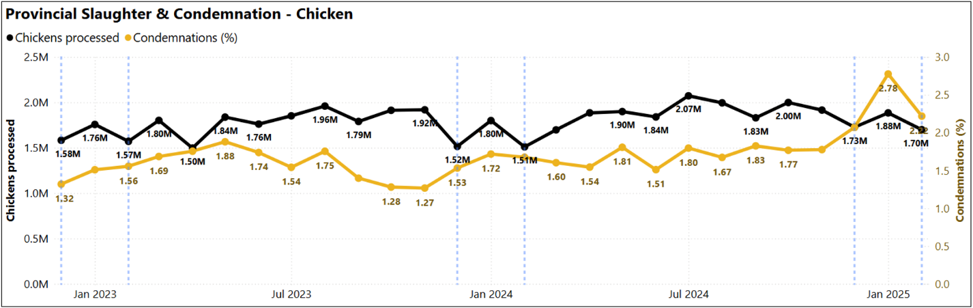
Chickens processed and condemnation percentage (December 2022-February 2025)
Federal slaughter plants: Number of chickens slaughtered by province, a) Jan-Dec 2024 and b) January-February 2025 (CAHSS Federally Inspected Slaughter Data).
a)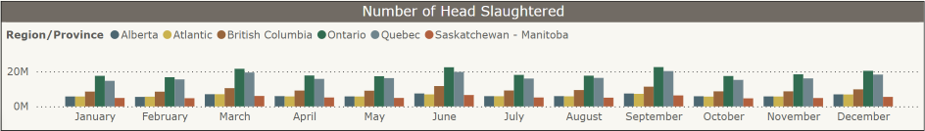 b)
b) 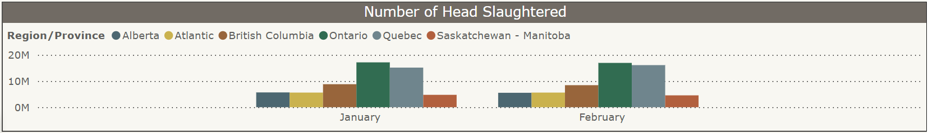
Top 10 chicken postmortem condemnation conditions per 10,000 slaughtered by province, Jan-Dec 2024 (CAHSS Federally Inspected Slaughter Data).
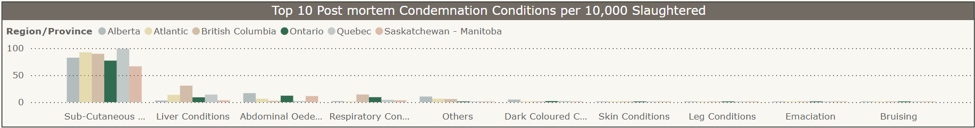
Subcutaneous conditions, abdominal edema, and respiratory conditions were reported as the main conditions for condemnation for chickens slaughtered in federally inspected plants in Ontario in 2024 (dark green bars).
Broiler-Breeders
Most of the conditions were stable this quarter with predominant bacterial lameness associated with wet litter due to cold winter conditions.
Practitioners
Stable conditions: fowl cholera, early bacterial infection ( E.coli, S. aureus and E. cecorum), other causes of mortality, lameness developmental/viral/nutritional, in-lay bacterial septicemia (E. coli, G. anatis and E. cecorum), IBV-sudden spike in mortality, necrotic enteritis, coccidiosis, fowl pox, ILT, salmonellosis, disease related to hatchability issue, aggression and cannibalism, multi-drug resistant E. coli (resistant to >3 drugs), histomoniasis and pre-lay mortality.
Stable to slightly increased conditions: CAV, IBV decreased production/egg abnormality, Mycoplasma, avian influenza and aMPV.
Equally stable and increased conditions: lameness bacterial (S. aureus, E. cecorum and E. coli).
Other conditions diagnosed: fatty liver syndrome used to be seen but suspected to be associated to pushing feed into production. See this less if bird weight is managed.
The following graph displays the frequency of the conditions seen in the flocks for the past 3 quarters. The most frequent 3 conditions seen this quarter were: lameness of bacterial origin, IBV decreased egg production/abnormal eggs, equally frequent- early systemic bacterial infection and in-lay systemic septicemia.
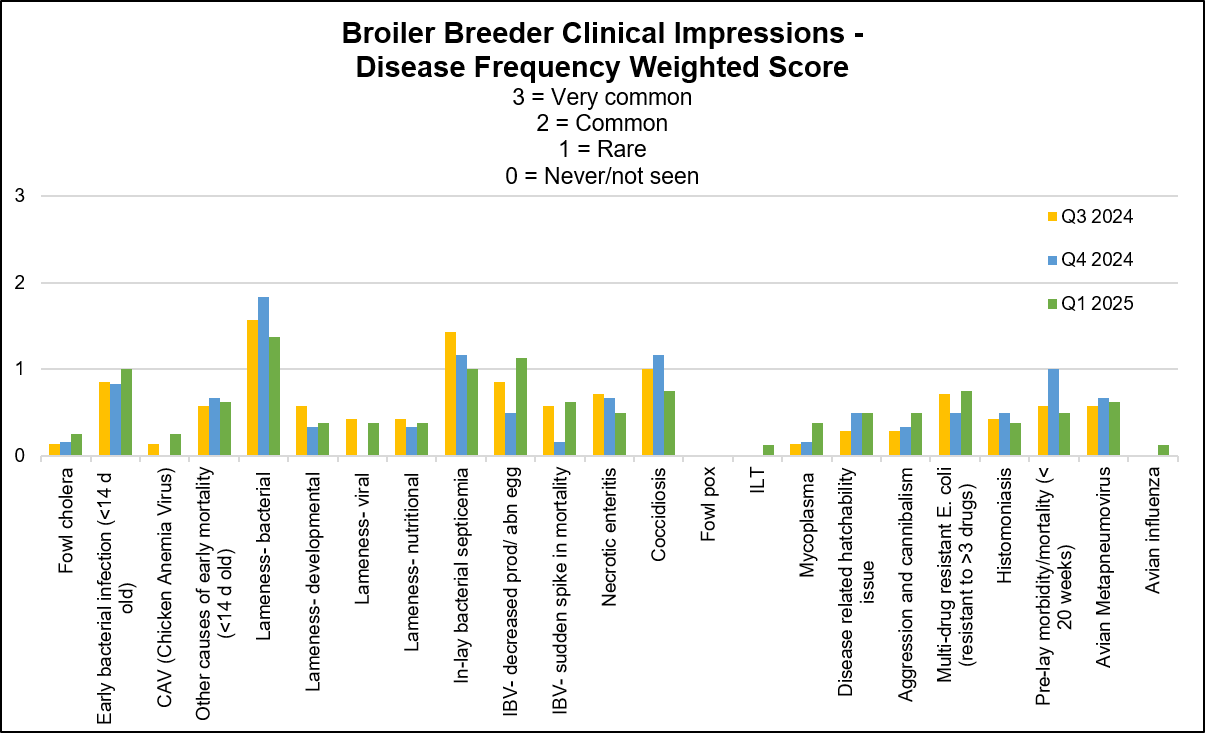
AHL
Fowl cholera (Pasteurella multocida) – Occasionally isolated from lameness cases from vaccinated flocks.
Similar number of cases (from previous quarter):
- Prelay morbidity/mortality (<20 weeks) – E. coli and S. aureus were isolated in pure culture or in combination. E. coli was also isolated with E. cecorum. Other diagnoses included intussusception, thymic atrophy and bursal atrophy/IBDV.
- Avian metapneumovirus (Type B and Type A).
- Lameness viral – Reovirus (1 case PCR).
- Necrotic enteritis (1 case C. perfringens isolated with E. coli).
- Intestinal parasitism – Histomonas.
Increased number of cases (from previous quarter):
- Early bacterial infection (<14 days old) – Cases of septicemia had E. coli and S. aureus isolated in pure culture. E. coli and E. cecorum were isolated together as well as in combination with S. aureus. Cases of yolk sacculitis had E. coli isolated in pure culture or in combination with P. aeruginosa.
- Other causes of mortality (> 14 days old) – Dehydration and starve out.
- Inlay bacterial septicemia – E. coli and S. aureus were isolated in pure culture, together and in combination with G. anatis. E. coli and E. cecorum were isolated together as well as in combination with S. aureus or C. perfringens.
Decreased number of cases (from previous quarter):
- Cellulitis/Dermatitis – E. coli, S. aureus and E. cecorum are isolated together.
- Bacterial pneumonia – S. aureus and E. coli together as well as with G. anatis.
- Lameness developmental (valgus deformity).
- Trauma (tendon hemorrhage).
- Lameness bacterial – S. aureus, and E. coli were each isolated in pure culture or in combination. S. aureus was also isolated with E. cecorum or S. hyicus. E. coli and E. cecorum were also isolated together and in combination with S. aureus. A case of osteomyelitis had E. coli and S. aureus isolated. There were also 2 cases of vertebral osteomyelitis having E. coli isolated alone or in combination with E. cecorum.
- Coccidiosis – Identified in the small intestines and ceca.
- IBV (decreased productivity/abnormal eggs/sudden spike in mortality) – (IBV_DMV_ON_21-017385, IBV_DMV_ON_15-077145, IBV_CA_1734_04_ON_12-025379, IBV_Mass-MA5 vaccine, and IBV_Conn vaccine).
- Other causes of inlay mortality – Feed impaction.
- Urate nephrosis/visceral urates/articular urates.
- Amyloidosis.
No cases diagnosed this quarter:
- Lameness developmental (tibial dyschondroplasia).
- Lameness nutritional (rickets).
- Pododermatitis.
- White Chick Syndrome.
- Fowl pox.
- ILT and Mycoplasmosis.
Other diagnostic findings:
- Gangrenous dermatitis (C. septicum / S. aureus, Clostridium sp. / S. aureus, Clostridium sp. / E. coli / E. cecorum), neoplasia (leiomyoma, ovarian), salpingitis, corneal ulceration / keratitis / uveitis, vaccine reaction, granulomatous & interstitial nephritis, subcutaneous edema, enteritis, ulcerative typhlitis with cecal cores, deep pectoral myopathy and bile duct hyperplasia.
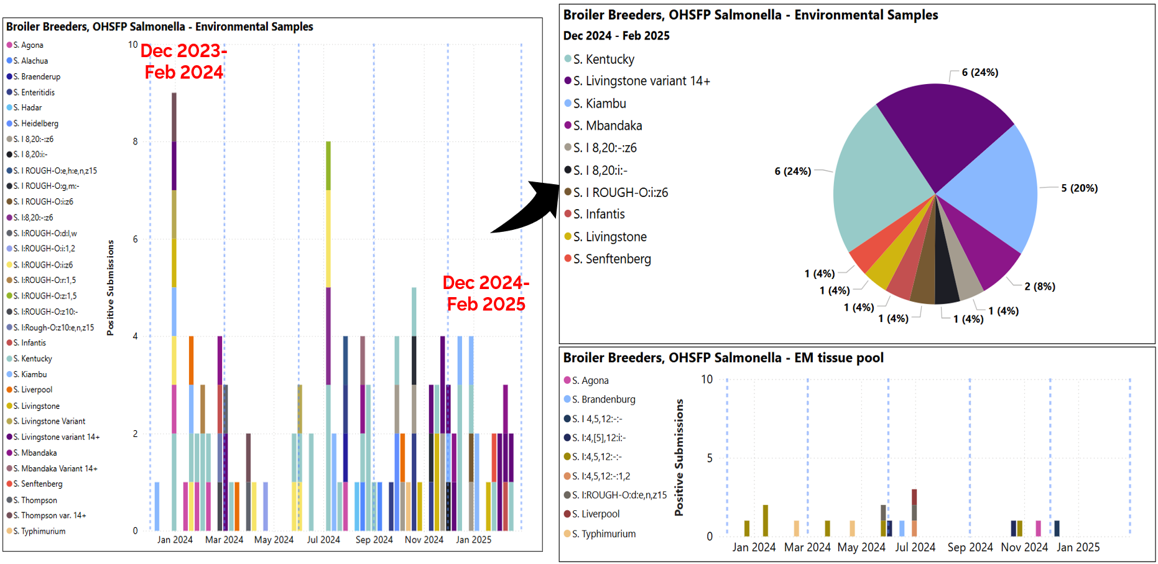
- Animal Health Laboratory Salmonella Isolations in Broiler Breeders (see graph below, blue dashed lines indicate quarter periods Dec-Feb, Mar-May, Jun-Aug, Sept-Nov):
- No positive submissions outside of OHSFP testing (Ontario Hatchery and Supply Flock Program).
- OHSFP environmental samples – S. Kentucky, Livingstone var 14+, and Kiambu most isolated (top right graph showing Dec-Feb isolations only).
- One OHSFP excess mortality submission – S. I 4,5,12:-:- isolated (bottom right graph).
Layers
Veterinarians are reporting good, stable and solid health status of the flocks. There were more S. Enteritidis detections in the environment reported this quarter.
Practitioners
Stable conditions: osteoporosis, bacterial peritonitis/salpingitis (E. coli, S. aureus and S. hyicus), early systemic bacterial infection, other causes of mortality, focal duodenal necrosis (FDN), IBV-production/abnormal egg, respiratory issues, salmonellosis, ILT, mycoplasmosis, coccidiosis, necrotic enteritis, hysteria, multi-drug resistant E. coli (resistant to > 3 drugs), histomoniasis and avian influenza.
Stable to slightly increased conditions: aMPV infections, aggression and cannibalism. Below graph depicts the frequency of the conditions seen in the flocks for the past 3 quarters. The most frequent 3 conditions seen this quarter were: bacterial salpingitis/peritonitis, osteoporosis, equally frequent- IBV- production drop/abnormal eggs, aMPV, aggression and cannibalism.
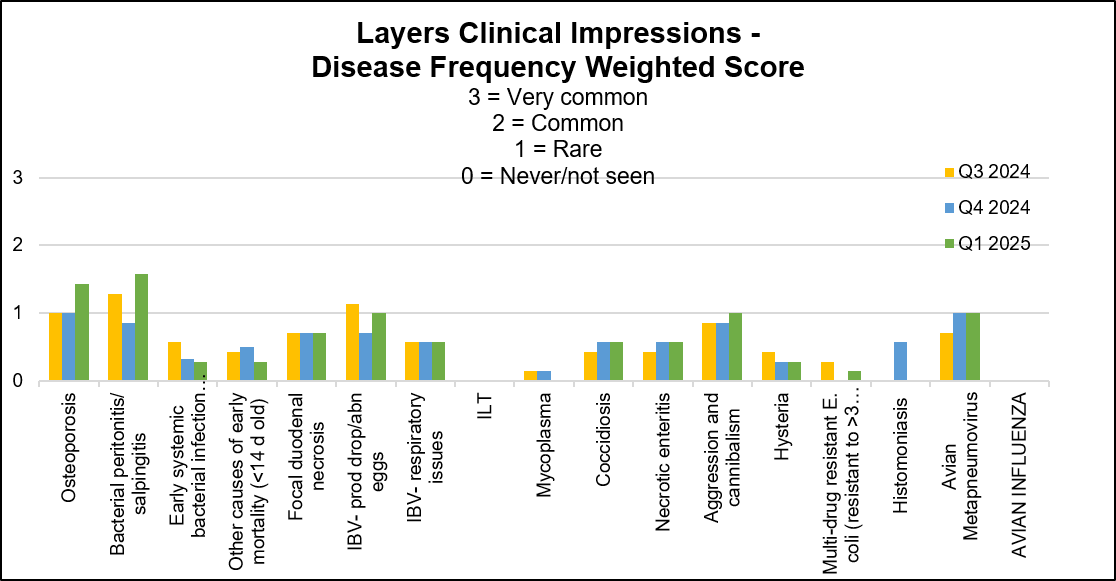
AHL
Similar number of cases (from previous quarter):
- Infectious bronchitis (IBV – production drop/egg abnormalities/ respiratory) – (IBV_DMV_ON_15-077145 and IBV_Mass-MA5 vaccine).
Increased number of cases (from previous quarter):
- Early mortality (other) – E. coli septicemia, tracheitis, and pulmonary congestion / edema.
Decreased number of cases (from previous quarter):
- Prelay mortality (<20 weeks old) – Septicemia, dehydration, off feed, aspergillosis, tracheitis (mild) as well as severe bursal atrophy, bursitis and intralesional bacteria.
- In-lay mortality (>20 weeks old) – Renal abscessation with bacteria and sepsis.
No cases diagnosed this quarter:
- Early mortality (<14 days old, starveouts).
- Early mortality (<14 days old, omphalitis).
- Osteoporosis.
- Leg issues – Trauma, bone fracture.
- Leg issues – Bacterial.
- Leg issues (other).
- Focal duodenal necrosis (FDN).
- Necrotic enteritis.
- Coccidiosis.
- Intestinal parasitism (other).
- Avian metapneumovirus.
- Aggression/Hysteria.
- ILT.
- Mycoplasma.
- Elevated DOA.
Other diagnostic findings:
Idiopathic production loss.
Salmonellosis (clinical) / Salmonella isolation
- Animal Health Laboratory Salmonella Isolations (graphs below, blue dashed lines indicate quarter periods Dec-Feb, Mar-May, Jun-Aug, Sept-Nov):
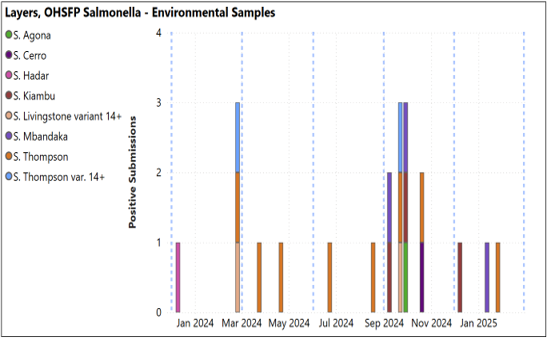
- Layers: No positive submissions outside of OHSFP. OHSFP submissions with S. Thompson, Mbandaka, and Kiambu isolated. No non-environmental OHSFP samples positive (below, left).
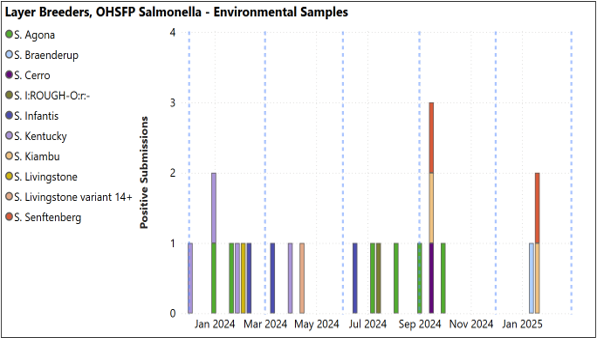
- Layer breeders: No positive samples outside of OHSFP. OHSFP with S Kiambu, Seftenberg and Braenderup isolated. No non-environmental OHSFP samples positive (below, right).
Turkeys
Practitioners
Turkey flocks continue to have health challenges with aMPV type B infection listed as the main condition and it was detected in smaller and multiage facilities. Flocks are presenting increased percentage of mortality, and many have clinical signs related to secondary E. coli infections. Same as previous quarter, practitioners are reporting that unaffected flocks appear in good health and with great performance.
Stable conditions: other causes of mortality and necrotic enteritis.
Stable to slightly decreased conditions: fowl cholera, erysipelas, early systemic infection, mycotic respiratory disease, mycoplasmosis, salmonellosis, reovirus tenosynovitis, round heart and histomoniasis.
Stable to slightly increased conditions: late systemic bacterial infection (E. coli and S. aureus), ORT, other respiratory disease, enteritis, multi drug resistant E. coli (resistant to > 3 drugs), aggression and cannibalism.
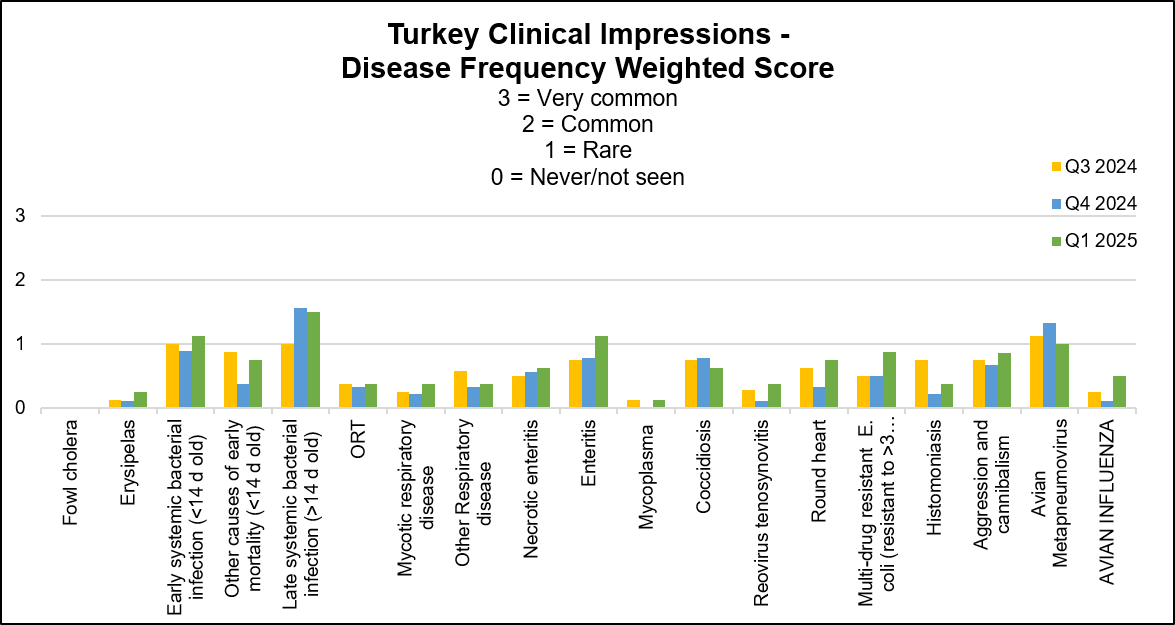
Stable to increased conditions: cases of aMPV and avian influenza. Coccidiosis was mainly stable with equal number of respondents reporting increased and decreased cases this quarter. Aortic rupture was listed as another condition and was reported stable to slightly increased. The following graph displays the frequency of the conditions seen in the flocks for the past 3 quarters. The most frequent 3 conditions seen this quarter were: late systemic bacterial infection, early systemic bacterial infection and enteritis.
AHL
Similar number of cases (from previous quarter):
- Necrotic enteritis.
- Reovirus – PA 01769-14.
Increased number of cases (from previous quarter):
- Bacterial pneumonia – Airsacculitis
- Enteric disease – Enteritis and S. Hadar.
- Parasitism – Ascaridia.
Decreased number of cases (from previous quarter):
- Early systemic bacterial infection (<14 days old) – E. coli isolated in pure culture.
- Late systemic bacterial infection (>14 d old) – E. coli isolated in pure culture.
- Avian metapneumovirus – Type B
No cases diagnosed this quarter:
- Fowl cholera (Pasteurella multocida).
- Erysipelas.
- Other causes of early mortality (<14 days old, starvout / dehydration).
- ORT (Ornithobacterium rhinotracheale).
- Mycotic respiratory disease.
- Coccidiosis.
- Histomoniasis.
- Tibial dyschondroplasia.
- Mycoplasma.
- Rickets.
- Condemnations.
- DOA.
Other diagnostic findings:
Botulism Type C (PCR), gizzard mucosal necrosis and hemorrhage, heterophilic enteritis and typhlitis, steatitis and urate nephrosis.
Salmonellosis (clinical)/Salmonella isolation:
Practitioners: Reported stable to slightly increased. S. Hadar, S. Newport, S. Albany and S. Montevideo were listed as the main isolates.
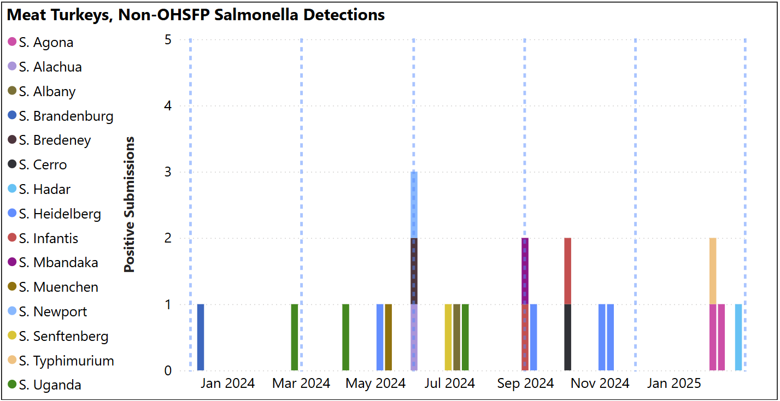
Animal Health Laboratory Salmonella Isolations: Turkey – Meat S. Agona, Typhimurium and Hadar isolated (see below, blue dashed lines indicate quarter periods Dec-Feb, Mar-May, Jun-Aug, Sept-Nov).
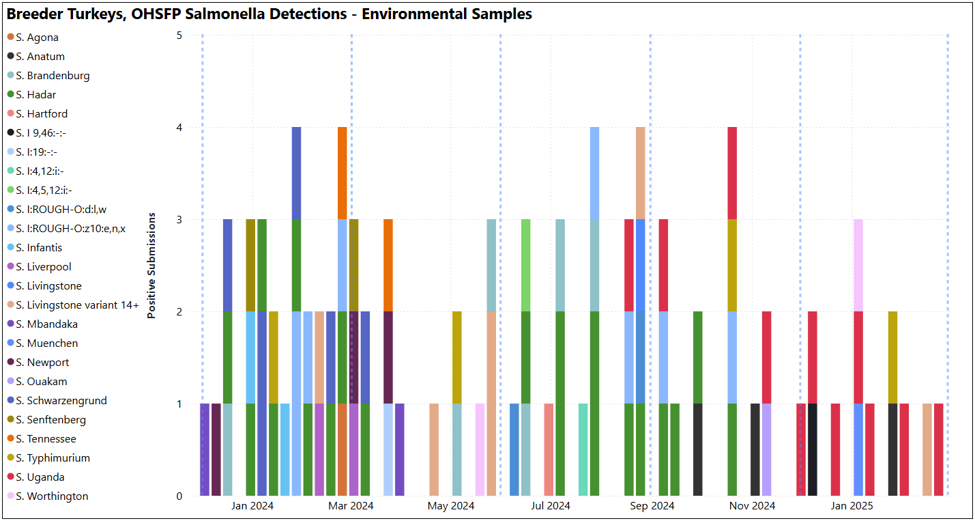
Animal Health Laboratory Salmonella Isolations: Turkey – breeders OHSFP – All environmental samples this quarter, S. Uganda (shown as red bars below) was the most isolated (7/13 detections in graph below. Blue dashed lines indicate quarter periods: Dec-Feb, Mar-May, Jun-Aug, Sept-Nov). Non-OHSFP – 4 detections over the quarter, S. Javiana and S. Agona isolated (not shown).
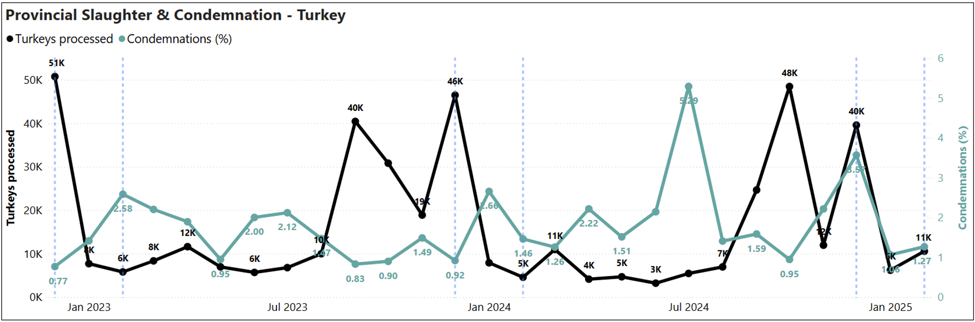
Provincial slaughter plants condemnations: Turkey slaughtered and percentage of condemnation in provincial plants (Dec 2022-Feb 2025)
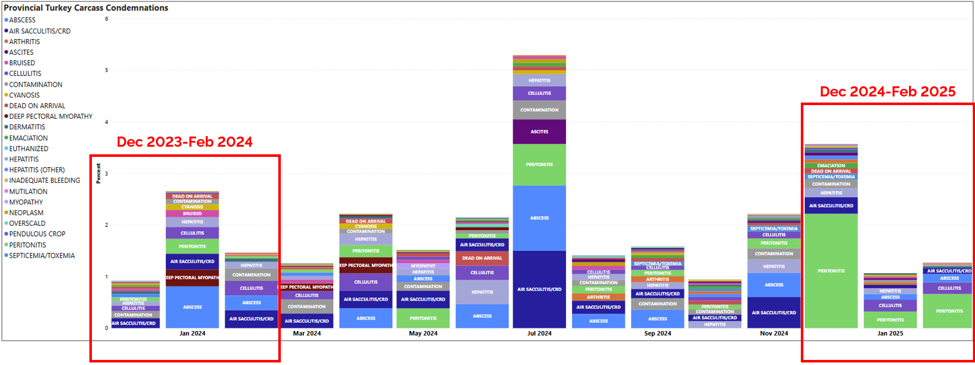
Turkey condemnation conditions (Dec 2023-Feb 2025)
 Federal slaughter plants: Number of turkeys slaughtered by region, a) Jan-Dec 2024 and b) Jan-Feb 2025. From CAHSS Federally Inspected Slaughter Data.
Federal slaughter plants: Number of turkeys slaughtered by region, a) Jan-Dec 2024 and b) Jan-Feb 2025. From CAHSS Federally Inspected Slaughter Data.
a)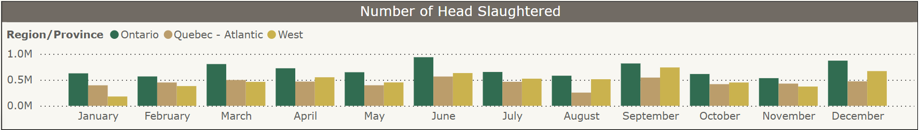 b)
b) 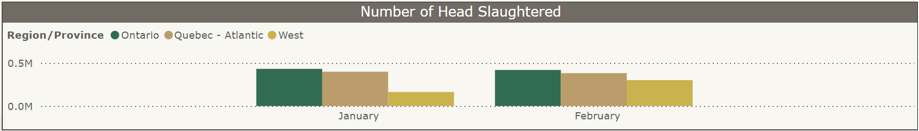
Top 10 turkey post-mortem condemnation conditions per 10,000 slaughtered, Jan-Dec 2024 (CAHSS Federally Inspected Slaughter Data).
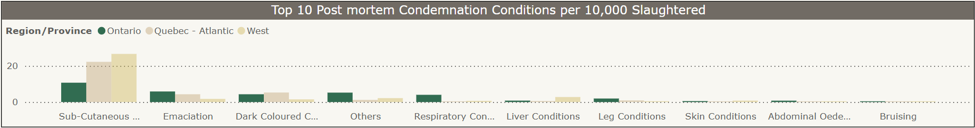
Subcutaneous conditions, emaciation, and “other” conditions were reported as the main conditions for condemnation for turkeys slaughtered in federally inspected plants in Ontario in 2024.
Small Flock
Chickens: AHL: Diagnoses at AHL included lice, mites (Knemidocoptes), intestinal parasitism (heterakis, nematodes (Ascaridia), cestodes), Marek’s disease, neoplasia (adenocarcinoma), salpingitis, yolk peritonitis, bacterial septicemia (P. multocida), urate nephrosis, M. gallisepticum / E. coli / E. cecorum, cannibalism, IBV (suspicious on histology), IBV (PCR, IBV_DMV_ON_15-077145), emaciation, humeral fracture with cellulitis, angular limb deformity (metabolic bone disease), egg bound, ovarian hemorrhage, ingluvitis, subcutaneous hemorrhage caudal skull, necrotizing cloacitis, fibrinous and caseous conjunctivitis (Avibacterium spp.) and aMPV PCR (Type B). ILT: 1 case (CEO/TCO vaccine-like strain TCAA cluster). Gamebird/Ratities: AHL: Three quail cases submitted this quarter. Quail: 1) Mycotic pneumonia. 2) E. coli. 3) Proliferative blepharitis/ follicular cysts / conjunctivitis, salpingitis, leiomyoma. Waterfowl: AHL: Seven duck and 1 mute swan case submitted this quarter. Mute Swan: 1) Carpometacarpal, rib and spinal fractures. Focally extensive myelomalacia and hemorrhage. Ducks: 1) Avian Influenza. 2) Ammonia burn (cornea). 3) Fatty liver. 4) Muscovy – Emaciation, ascites. 5) Ecto-parasitism and endo-parasitism. 6) Muscovy – Amyloidosis, septicemia (Streptococcus uberis, E. coli), valvular endocarditis, myocardial fibrosis). 7) Duck (Ancona) – Toxicity (grocery store waste). Practitioners: Information provided with the clinical impression in chickens only: Frequency of conditions seen in the practice: Common to rare: ectoparasitism, pododermatitis. Rare to not seen: ascites, intestinal parasitism, trauma, peritonitis/salpingitis, frost bite and beak deformities. Not seen: Marek’s disease, other neoplasia, GI impaction, vent trauma, bacterial septicemia, pneumonia/airsacculitis, FLHS, urate nephrosis/ visceral urates, articular urate deposits, ILT, histomoniasis, erysipelas, toxins, mycoplasma and aMPV. Changes since last quarter: stable pododermatitis and ectoparasitism, increased frost bite and beak deformity.
Poultry research from Ontario and beyond
Events and News
Poultry Industry Council events: https://www.poultryindustrycouncil.ca/events Poultry Health Research Network information, events, and lectures can be accessed on the PHRN website: https://phrn.net/ or on the PHRN YouTube channel: https://www.youtube.com/user/PoultryHRN
Thank You! We thank the following poultry veterinarians who completed the veterinary survey: Dr. Tim Abolarin, Dr. Elizabeth Black, Dr. Joanne Dias, Dr. Daniella Di Pirro, Dr. Shahbaz Haq, Dr. Elana Huong, Dr. Anastasia Novy, Dr. Mike Petrik, Dr. Joanne Rafuse, Dr. Nahal Ramezani, Dr. Ben Schlegel, Dr. Chanelle Taylor, Dr. Brenna Tuer, Dr. Alex Weisz, and Dr. Jessalyn Walkey.


